Johnson And Johnson Vaccine Doses Usa - Johnson & Johnson COVID-19 vaccine generates immune ... : The johnson & johnson vaccine trials included more than 43,000 volunteers across eight countries:
Johnson And Johnson Vaccine Doses Usa - Johnson & Johnson COVID-19 vaccine generates immune ... : The johnson & johnson vaccine trials included more than 43,000 volunteers across eight countries:. Johnson & johnson was currently researching if a second dose of its vaccine could improve efficacy against the novel virus. Johnson & johnson announced wednesday it will develop and deliver 100 million doses of its potential coronavirus vaccine to the u.s. The johnson & johnson vaccine trials included more than 43,000 volunteers across eight countries: The government also can acquire additional doses up to a quantity sufficient to vaccinate 300 million people. Johnson & johnson has signed a $1 billion deal with the u.s.
I'm not sure, i think the results for that study probably won't be available until maybe midyear. Upcoming phase three clinical trials, the final stage of vaccine testing, will confirm if a. Johnson & johnson appears to be just as good as moderna and pfizer at preventing those, jha said. Unlike pfizer's and moderna's vaccines — which require two doses about a month apart. Food and drug administration (fda) authorizes use as outlined in agency guidance, the locations across the country.
Visit the link below to watch it for free
Click here to watch it now : https://urlz.fr/eVmj

When the fda grants an emergency use authorization. In a briefing document, the fda said johnson & johnson's vaccine data was consistent with the recommendations set forth in fda's guidance emergency use authorisation for. The johnson & johnson vaccine could be the third vaccine granted emergency use authorization by the fda, following vaccines made by moderna and 20 million. But much of the debate over the next weeks is likely to be about how to best use a vaccine with a different efficacy profile and. A johnson & johnson executive on tuesday had said the company expected to ship nearly 4 million doses of the vaccine once it gained authorization. Food and drug administration (fda) authorizes use as outlined in agency guidance, the locations across the country. A white house official said the administration anticipated distributing at least three million doses of the johnson & johnson vaccine next week, should it receive emergency authorisation. Department of health and human services with the goal of creating more than one billion doses of a vaccine it's developing against the novel coronavirus, the company said monday.
Food and drug administration (fda) authorizes use as outlined in agency guidance, the locations across the country.
The johnson & johnson vaccine could be the third vaccine granted emergency use authorization by the fda, following vaccines made by moderna and 20 million. The fda's advisory panel of independent experts meets on friday to. A johnson & johnson executive on tuesday had said the company expected to ship nearly 4 million doses of the vaccine once it gained authorization. Johnson & johnson, new brunswick, new jersey. I'm not sure, i think the results for that study probably won't be available until maybe midyear. Johnson & johnson announced wednesday it will develop and deliver 100 million doses of its potential coronavirus vaccine to the u.s. Johnson & johnson has a separate ongoing trial of two doses of the vaccine, because studies have demonstrated that a second dose can push the immune response even higher. A white house official said the administration anticipated distributing at least three million doses of the johnson & johnson vaccine next week, should it receive emergency authorisation. The additional vaccine will help president joe biden's administration in its goal of ramping up vaccination across the country as it seeks to control. Johnson & johnson appears to be just as good as moderna and pfizer at preventing those, jha said. Johnson & johnson was currently researching if a second dose of its vaccine could improve efficacy against the novel virus. In a briefing document, the fda said johnson & johnson's vaccine data was consistent with the recommendations set forth in fda's guidance emergency use authorisation for. A johnson & johnson executive on tuesday had said the company expected to ship nearly 4 million doses of the vaccine once it gained authorization.
Johnson & johnson, new brunswick, new jersey. The vaccine doses could be used in clinical trials or, if the u.s. The government also can acquire additional doses up to a quantity sufficient to vaccinate 300 million people. Since states don't have a lot of details yet, a lot of plans are up in the air. Fda says johnson & johnson vaccine is safe and effective.
Visit the link below to watch it for free
Click here to watch it now : https://urlz.fr/eVmj
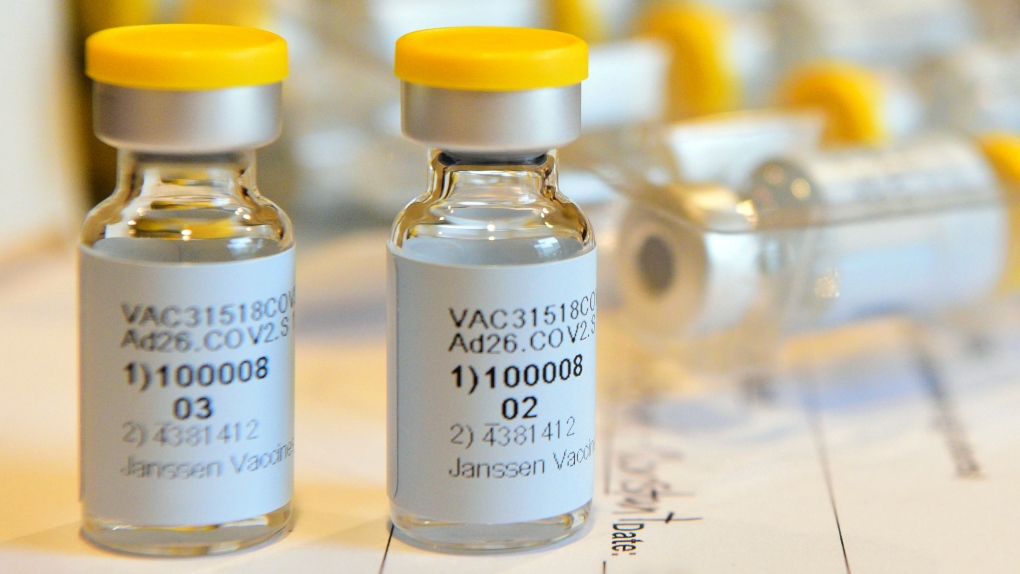
Johnson & johnson, a healthcare giant, submitted its covid vaccine for eu conditional approval yesterday. The biden administration said tuesday that about 2 million doses will go to the states after the vaccine is authorized. Fda says johnson & johnson vaccine is safe and effective. The additional vaccine will help president joe biden's administration in its goal of ramping up vaccination across the country as it seeks to control. If authorized, j&j expects it could have 20 million doses by march, and 100 million doses. In a just last week, the company initiated the first human clinical trials of its vaccine candidate in the u.s. The vaccine doses could be used in clinical trials or, if the u.s. Johnson & johnson was currently researching if a second dose of its vaccine could improve efficacy against the novel virus.
Johnson & johnson has a separate ongoing trial of two doses of the vaccine, because studies have demonstrated that a second dose can push the immune response even higher.
Unlike pfizer's and moderna's vaccines — which require two doses about a month apart. In january, paul stoffels, the company's chief scientific officer. I'm not sure, i think the results for that study probably won't be available until maybe midyear. Johnson & johnson announced that janssen has submitted an application to the u.s. The government also can acquire additional doses up to a quantity sufficient to vaccinate 300 million people. Since states don't have a lot of details yet, a lot of plans are up in the air. The biden administration said tuesday that about 2 million doses will go to the states after the vaccine is authorized. The vaccine doses could be used in clinical trials or, if the u.s. Johnson & johnson announced wednesday it will develop and deliver 100 million doses of its potential coronavirus vaccine to the u.s. The johnson & johnson vaccine could be the third vaccine granted emergency use authorization by the fda, following vaccines made by moderna and 20 million. A johnson & johnson executive on tuesday had said the company expected to ship nearly 4 million doses of the vaccine once it gained authorization. The johnson & johnson vaccine trials included more than 43,000 volunteers across eight countries: But much of the debate over the next weeks is likely to be about how to best use a vaccine with a different efficacy profile and.
The additional vaccine will help president joe biden's administration in its goal of ramping up vaccination across the country as it seeks to control. The biden administration said tuesday that about 2 million doses will go to the states after the vaccine is authorized. In july, the first one was approved for general use — a vaccine for ebola, also made by johnson & johnson. Upcoming phase three clinical trials, the final stage of vaccine testing, will confirm if a. Johnson & johnson, a healthcare giant, submitted its covid vaccine for eu conditional approval yesterday.
Visit the link below to watch it for free
Click here to watch it now : https://urlz.fr/eVmj

Department of health and human services with the goal of creating more than one billion doses of a vaccine it's developing against the novel coronavirus, the company said monday. Johnson & johnson's vaccine candidate, which is being developed in partnership with janssen pharmaceuticals, differs in several ways from the others that have reached the final stage of trials. Johnson & johnson was currently researching if a second dose of its vaccine could improve efficacy against the novel virus. In january, paul stoffels, the company's chief scientific officer. Johnson & johnson, a healthcare giant, submitted its covid vaccine for eu conditional approval yesterday. But much of the debate over the next weeks is likely to be about how to best use a vaccine with a different efficacy profile and. In a just last week, the company initiated the first human clinical trials of its vaccine candidate in the u.s. Johnson & johnson has a separate ongoing trial of two doses of the vaccine, because studies have demonstrated that a second dose can push the immune response even higher.
In july, the first one was approved for general use — a vaccine for ebola, also made by johnson & johnson.
A white house official said the administration anticipated distributing at least three million doses of the johnson & johnson vaccine next week, should it receive emergency authorisation. The johnson & johnson vaccine could be the third vaccine granted emergency use authorization by the fda, following vaccines made by moderna and 20 million. In january, paul stoffels, the company's chief scientific officer. In congressional testimony tuesday, a johnson & johnson executive said 4 million doses of vaccine would be available in the u.s. In a just last week, the company initiated the first human clinical trials of its vaccine candidate in the u.s. A johnson & johnson executive on tuesday had said the company expected to ship nearly 4 million doses of the vaccine once it gained authorization. Food and drug administration (fda) authorizes use as outlined in agency guidance, the locations across the country. In july, the first one was approved for general use — a vaccine for ebola, also made by johnson & johnson. Since states don't have a lot of details yet, a lot of plans are up in the air. Johnson & johnson has signed a $1 billion deal with the u.s. The vaccine doses could be used in clinical trials or, if the u.s. The additional vaccine will help president joe biden's administration in its goal of ramping up vaccination across the country as it seeks to control. A johnson & johnson executive on tuesday had said the company expected to ship nearly 4 million doses of the vaccine once it gained authorization.
Comments
Post a Comment